


EUDAMED is the IT system developed by the European Commission to implement Regulation (EU) 2017/745 on medical devices and Regulation (EU) 2017/746 on in-vitro diagnostics medical devices including the actor roles.
The European Commission defines an Actor as:
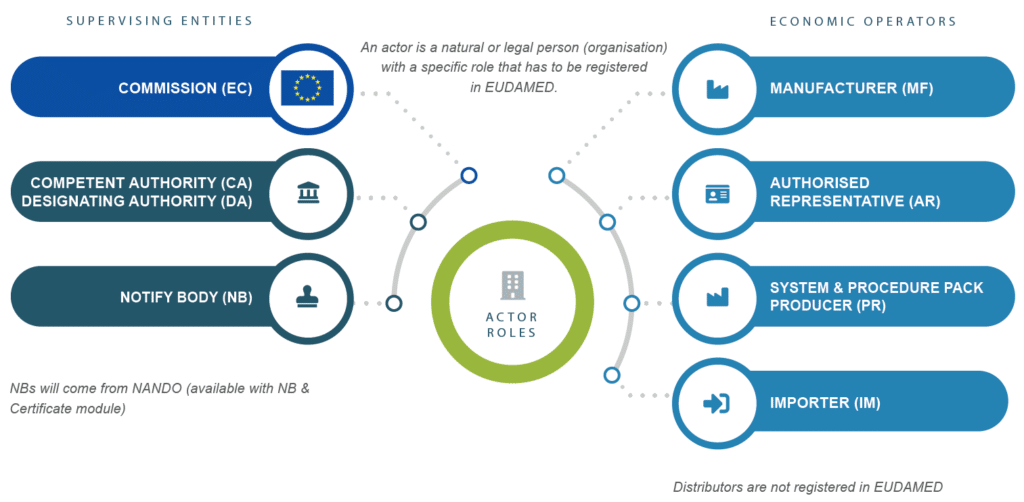
“… a natural or legal person with a specific role that has to be registered in EUDAMED.”
Thus, the regulations MDR and IVDR define those roles, or economic operators, which are subject to registration in EUDAMED.
Registration in the Actor Module of EUDAMED (module 1) is required for all economic operators or actors falling under the regulation 2017/745 (MDR) and/or 2017/746 (IVDR). There is no need, however, to register an actor when the product is certified and compliant with the currently valid directives, i.e. 93/42/EC (MDD), 98/79/EC (IVD), or 90/385/EV (AIMD).
Registration is mandatory for the following actors and roles:
Clearly said, distributors have not to be registered in EUDAMED.
On the authority side, the Competent Authorities (CA), Designated Authorities (DA), and Notified Bodies (NB) need to register as well. For NBs, there will be a separate module, the NB and Certificate module and NBs will come from the NANDO database.
Under the MDR and IVDR, the actor registration in EUDAMED becomes a requirement. Some of the data need to be uploaded prior marketing of the product (data required under module 1 to 4) and others need to be curated over time (data required under modules 5 and 6). Not complying to these requirements will most probably lead to the rejection or denial of a market authorization.
The registration of the actors is a straight forward process, whereas each actor registers itself in EUDAMED, the supervising and relevant CA will validate the data and if deemed acceptable, the Single Registration Number (SRN) is granted. The SRN is emailed then to the actor or economic operator.
For non-EU manufacturers, the EC REP will first check on the submitted data, prior to the relevant CA validates the data and grants the SRN.
Courtesy: DG Health and Safety
Avanti Europe’s Experts have a decade-long track record and expertise in consulting and contracting to the Medical Device industry. Our experts support your company with hands-on workforce and support the notification and data entry into EUDAMED, definition of the QMS, the labeling of the product, processes, the documentation, training for the company staff, and audits for Medical Device QMS, and Supplier audits. Visit our online shop for checklists and other services.

This website uses cookies so that we can provide you with the best user experience possible. Cookie information is stored in your browser and performs functions such as recognising you when you return to our website and helping our team to understand which sections of the website you find most interesting and useful.
Strictly Necessary Cookie should be enabled at all times so that we can save your preferences for cookie settings.
If you disable this cookie, we will not be able to save your preferences. This means that every time you visit this website you will need to enable or disable cookies again.
More information about our Privacy Policy