First things first: Don’t get fooled about the Eudamed2 Database already being around in compliance with the Medical Device Directive MDD 93/42/EEC. The new EUDAMED database is actually an update and will be compliant with the Medical Device Regulation MDR 2017/745 and In-Vitro Device Regulation IVDR 2017/746. So, the EUDAMED is not a totally new concept.
EUDAMED is the IT system developed by the European Commission to implement Regulation (EU) 2017/745 on medical devices and Regulation (EU) 2017/746 on in-vitro diagnostics medical devices.
Why a new EUDAMED database?
With the update of the directives to regulations for both medical devices and in-vitro diagnostics, an update of the EUDAMED database became inevitable.
The database will be the single repository for data on medical devices and in-vitro diagnostics marketed in the EU/EEA/EFTA to publicly access safety and performance data along to the economic operators and Notified Bodies involved in the market clearance and surveillance of every specific product.
The European Commission further envisions a more transparent and congruent data collection over all medical devices and in-vitro diagnostics in its market. This should allow not only to identify easily the actors and responsible but also to monitor the safety and performance of products over time.
what data does EUDAMED collect?
The EUDAMED database is organized in modules. There are 6 modules, which are:
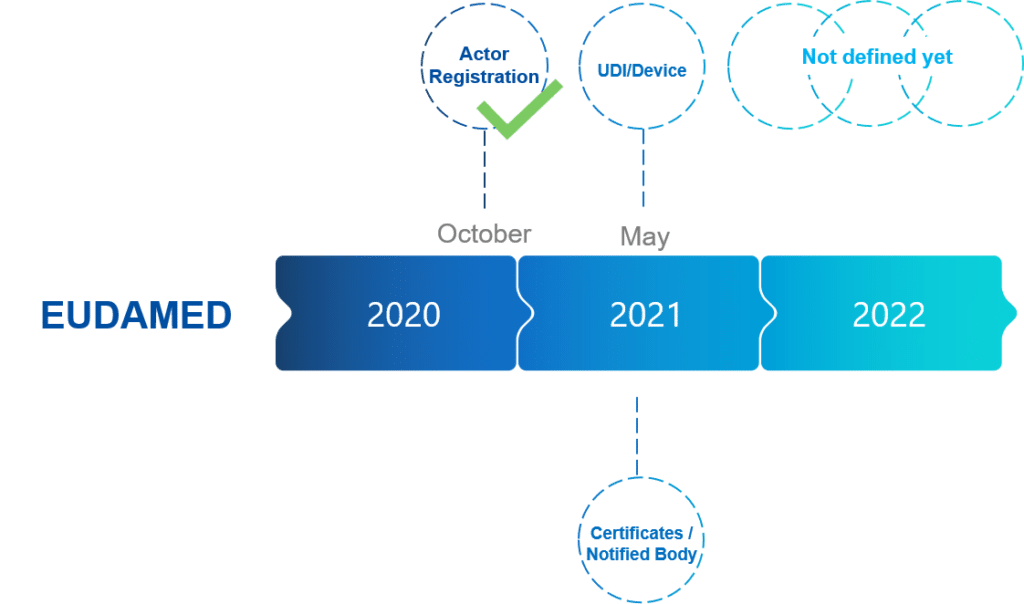
- Module 1: Actors (economic operators) registration
- Module 2: UDI/Devices registration
- Module 3: Notified Bodies and Certificates
- Module 4: Clinical Investigations and performance studies
- Module 5: Vigilance and post-market surveillance
- Module 6: Market Surveillance
These modules will be implemented over time and according to the implementation dates of the MDR and IVDR, as the European Commission promised. The individual modules will store the data crucial to identify the product, its actors, certificates granted and assigned to it as well as safety and performance data over time.
why should you care?
Under the MDR and IVDR, the data upload to EUDAMED becomes a requirement. Some of the data need to be uploaded prior marketing of the product (data required under module 1 to 4) and others need to be curated over time (data required under modules 5 and 6). Not complying to these requirements will most probably lead to the rejection or denial of a market authorization.
What is the timeline?
The implementation of the EUDAMED has been delayed and with the delay in the implementation of the MDR by 1 year, the EUDAMED is back on track. There are no distinct due dates given by the European Commission but months are indicated for the first 3 modules:
How Avanti Europe can help
Avanti Europe’s Experts have a decade-long track record and expertise in consulting and contracting to the Medical Device industry. Our experts support your company with hands-on workforce and support the notification and data entry into EUDAMED, definition of the QMS, the labeling of the product, processes, the documentation, training for the company staff, and audits for Medical Device QMS, and Supplier audits. Visit our online shop for checklists and other services.