understanding the classification rules
The In-Vitro Diagnostics Regulation (MDR) EU 2017/746 defines in its Annex VIII the classification rules for in-vitro diagnostics. Read here how these rules are broken down and find explanations on under which rules medical devices might fall.
the rules
The IVDR 2017/746 contains overall 7 rules and changes the classification assignment to A, B, C and D in contrast to the IVDD 98/79/EEC. The rules are grouped into the primary application of the in-vitro diagnostic. Selecting the applicable rule for the product is decisive of the risk classification.
But what happens if the IVD has more than one intended purpose? From a risk-centric point of view, this is an easy one; the intended purpose with the highest risk classification wins and assigns the rule. The same is true if multiple rules are applicable to the product. Let’s have a look than at the rules.
Rule 1: Devices with a purpose of the following:
- detection of a transmissible agent n order to assess their suitability for transfusion, transplantation or cell administration
- detection of a transmissible agent that causes a life-threatening disease with a high or suspected high risk of propagation
- determining the infectious load of a life-threatening disease with critical patient monitoring
Rule 2: Devices intended for blood grouping, or tissue typing to ensure the immunological compatibility for subsequent transfusion, transplantation or administration when detecting one of the following markers:
- ABO system
- Rhesus system
- Kell system
- Kidd system
- Duffy system
Rule 3 : Devices intended for:
- detecting the presence of a sexually transmitted agent
- detecting the presence in cerebrospinal fluid or blood of an infectious agent without a high or suspected high risk of propagation
- detecting the presence of an infectious agent in which erroneous results can cause death or severe disability
- pre-natal screening of women’s immune status towards transmissible agents
- determining infective disease at which an erroneous result would lead to an erroneous patient management which can cause death or severe disability
- used as companion diagnostics
- used for disease staging at which an erroneous result would lead to an erroneous patient management which can cause death or severe disability
- used in screening, diagnosis, or staging of cancer
- human genetic testing
- monitoring of levels of medicinal products which an erroneous result would lead to an erroneous patient management which can cause death or severe disability
- management of patients suffering from a life-threatening disease or condition
- screening for congenital disorders in the embryo or foetus
- screening for congenital disorders in new-born babies which failure to treat or detect which can cause death or severe disability
Rule 4: Devices intended for self-testing, including such for:
- the detection of pregnancy, for fertility testing and for determining cholesterol level, and devices for the detection of glucose, erythrocytes, leucocytes and bacteria in urine
Exempt and ruled in their own rights are devices intended for near-patient testing.
Rule 5: Low-risk devices, such as:
- products for general laboratory use, accessories which possess no critical characteristics, buffer solutions, washing solutions, and general culture media and histological stains
- instruments intended specifically to be used for in-vitro diagnostic procedures
- specimen receptacles
Rule 6: Rule 6 covers all other devices.
Rule 7: Devices which are controls without a quantitative or qualitative assigned value.
Risk classes of IVD's
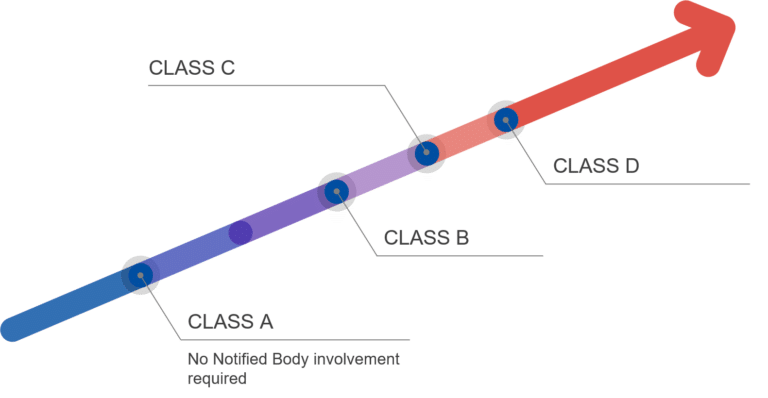
The IVDR 2017/746 understands the classification of medical devices as classifications of potential risk of harm to the patient. As Class A denote the lowest risk, Class D populates the other end of the risk range. Under these aspects, Class A in-vitro diagnosis medical devices don’t require the involvement of a Notified Body, whereas all other Classes do.
How Avanti Europe can help
Avanti Europe’s Experts have a decade-long track record and expertise in consulting and contracting to the MedTech and Pharma industry. Our experts support your company with hands-on workforce and support the definition of the medical device classification, the QMS, processes, the documentation and the training for the company staff. Visit our online shop for checklists and other services.