understanding the classification rules
The Medical Device Regulation (MDR) EU 2017/745 defines in its Annex VIII the classification rules for medical devices. Read here how these rules are broken down and find explanations on under which rules medical devices might fall.
the terms
The Annex VIII of the MDR defines some terms on the classification. These terms are:
- Transient means normally intended for continuous use for less than 60 minutes.
- Short term means normally intended for continuous use for between 60 minutes and 30 days
- Long term means normally intended for continuous use for more than 30 days
- Body orifice means any natural opening in the body, as well as the external surface of the eyeball, or any permanent artificial opening, such as a stoma
- Surgically invasive device means:
(a) an invasive device which penetrates inside the body through the surface of the body, including through mucous membranes of body orifices with the aid or in the context of a surgical operation; and
(b) a device which produces penetration other than through a body orifice - Reusable surgical instrument means an instrument intended for surgical use in cutting, drilling, sawing, scratching, scraping, clamping, retracting, clipping or similar procedures, without a connection to an active device and which is intended by the manufacturer to be reused after appropriate procedures such as cleaning, disinfection and sterilization have been carried out
- Active therapeutic device means any active device used, whether alone or in combination with other devices, to support, modify, replace or restore biological functions or structures with a view to treatment or alleviation of an illness, injury or disability
- Active device intended for diagnosis and monitoring means any active device used, whether alone or in combination with other devices, to supply information for detecting, diagnosing, monitoring or treating physiological conditions, states of health, illnesses or congenital deformities
- Injured skin or mucus membrane means an area of skin or a mucus membrane presenting a pathological change or change following disease or a wound
the rules
The MDR 2017/745 contains overall 22 rules and thus, 4 more than the previously published Medical Device Directive (MDD) 93/42/EC. The rules are grouped into the primary application of the medical device. Selecting the applicable rule for the medical device is decisive of the risk classification.
Rule 1-4 on non-invasive devices
Non-invasive devices are devices which barely are in contact with the patient by means of either being in contact with intact skin or not in contact with the patient at all.
Rule 1: Non-invasive devices
Rule 2: Non-invasive devices intended for channelling or storing (including blood, body fluids and cells)
Rule 3 : Non-invasive devices that modify the biological or chemical composition of blood, body liquids, and cells
Rule 4: Non-invasive devices in contact with injured skin or mucous membrane
Rule 5-8 on invasive devices
Invasive devices are devices which are introduced into the patient’s body by means of either through an opening in the body, so-called body orifice, or through a rupture or break of the skin.
Rule 5: Devices invasive through a body orifice
Rule 6: Surgically invasive devices for transient or impermanent use
Rule 7: Surgically invasive devices for short term use
Rule 8: Surgically invasive devices for long term use and implantable (including devices administering medicinal products, surgical meshes or spinal discs)
Rule 9-13 on active devices
An active device is any medical device relying on a source of power, either electrical energy or any source other than that directly generated by the human body or gravity.
Rule 9: Active therapeutic devices intended to exchange or administer energy
Rule 10: Active devices used for diagnosis and monitoring that emit ionizing radiation
Rule 11: Software intended to provide information which is used to make decisions on diagnosis or therapeutic purposes
Rule 12: Active devices intended to administer and/or remove medicinal products, body liquids or other substances
Rule 13: All other active devices
Rule 14-22 on special rules
Special rules extend the above set rules into the context of the potential features of the devices. As these features are special and do not fit into the rules above, the MDR has collated them into the special rules.
Rule 14: Devices incorporating a medicinal substance including human blood or plasma
Rule 15: Contraception or prevention of the transmission of sexually transmitted diseases
Rule 16: Specific disinfecting, cleaning, and rinsing devices
Rule 17: Devices specifically intended for recording of diagnostic images generated by X-rays
Rule 18: Devices utilizing non-viable tissues, cells of human origin, tissues of animal or derivatives thereof
Rule 19: Devices incorporating or consisting of nano-scale material
Rule 20: Invasive devices with respect to body orifices to administer medicinal products by inhalation
Rule 21: Substances or combinations of substances that are intended to be introduced into the human body via a body orifice or applied to the skin and that are absorbed
Rule 22: Active therapeutic devices with an integrated or incorporated diagnostic function which significantly determines the patient management
Even thought not explicitly named in the MDR 2017/745, the Class I medical devices can come as sterile, with a measuring function or re-usable. These classes of medical devices, often referred to as Class Is, Im, Ir, do require, in contract to Class I device, the involvement of a Notified Body.
Examples of devices per risk class
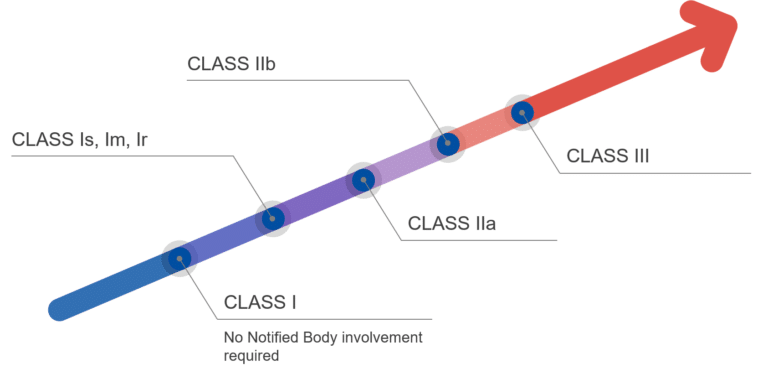
The MDR 2017/745 understands the classification of medical devices as classifications of potential risk of harm to the patient. As Class I denote the lowest risk, Class III populates the other end of the risk range. Under these aspects, Class I medical devices don’t require the involvement of a Notified Body, whereas all other Classes do.
How Avanti Europe can help
Avanti Europe’s Experts have a decade-long track record and expertise in consulting and contracting to the MedTech and Pharma industry. Our experts support your company with hands-on workforce and support the definition of the medical device classification, the QMS, processes, the documentation and the training for the company staff. Visit our online shop for checklists and other services.