the terms
… but first things first. Both terms relate to European Union (EU) regulation relating to combination products and first, let’s clarify on combination products to finally understand the terms. Combination products are products that are a combination of two or more differently regulated products. To get straight to the point, in the context of this article, combination products are the single-entity and integral combination of a medical device with a medicinal product in relation to the two terms consultation procedure and Notified Body opinion.
Conformity of MEdical devices
To assess the conformity of a medical device, a so-called Notified Body has to be involved which certifies the conformity of the product against the medical device regulation MDR 2017/745. This is a mandatory prerequisite for medical devices manufacturers before making such products available on the market.

In order to easily see that such a conformity assessment by a Notified Body has been successfully cleared, the manufacturer is allowed to affix the CE mark on the medical device. A medical device without a CE mark, it is either not a medical device or the medical device has not been cleared by a Notified Body for the market.
Drug device combination product
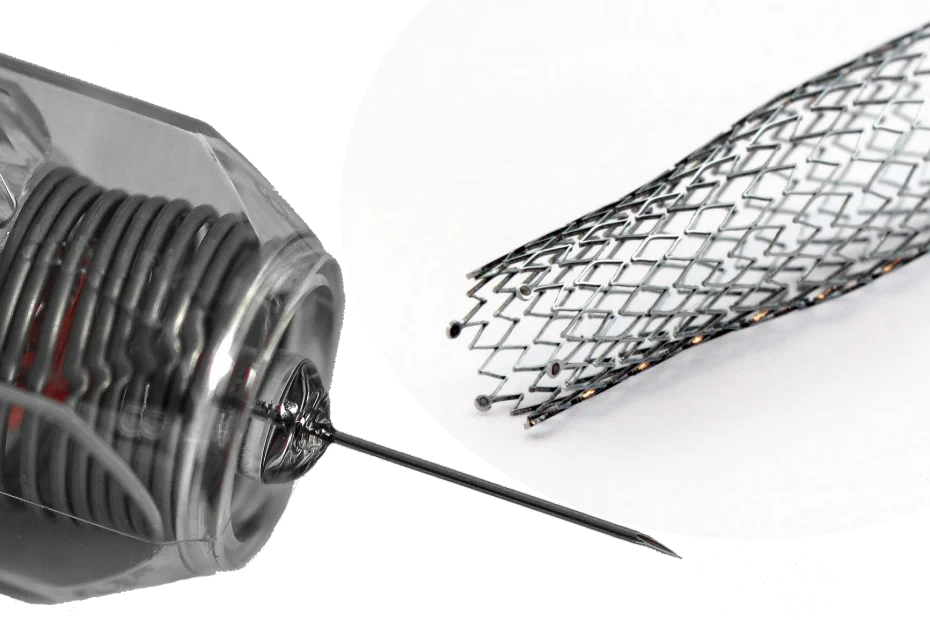
In terms of a combination product, also called drug-device combination product and sometimes related to as combined product (terms are used interchangeably in lieu of a clear definition in the regulation MDR 2017/745 as well as MPD 2001/83/EC), there is the possibility of having something between a medical device and another technical product. This “in-between physical object” is called medical device constituent part and forms the medical device part of a combination product. You got it! It is not a medical device, which a customer could actually buy at the pharmacy, and on the other hand, it is not just a technical product. It is something like a not-finished medical device. The medical device constituent part, as it is not a medical device, does not need to undergo conformity assessment and thus, will never be marked with the CE.
A medical device constituent part is combined with a drug product to form a medicinal product combination. Such products have a primary mode of action being either pharmacological, immunological or metabolic and thus, are not a medical device. Therefore, these products undergo Competent Authority review, based on the submission documentation, also called market authorization application (MAA). The Competent Authority, not in charge to review the technical part of the medical device constituent part will ask the market applicant to liaise with a Notified Body for technical review. The review of the conformity of a medical device constituent part in a Combination Product by a Notified Body is called “Notified Body Opinion”, abbreviated often as NBOp. Such a NBOp has to be submitted together with the MAA.

Drug Device Combination Products, per EU regulation, need to get a Notified Body Opinon.
Conformity of Pharmaceutical products
In terms of a combination product, API’s or drug substances are often used on or in a medical device to support the medical device function as a pharmaceutical, immunological or metabolic auxiliary action. Augmenting a medical device in such a way not only forms a combination product, but uses something else than a finished product without a market authorization. In many cases, generic substances or substances with a Pharmacopeial monograph in the Ph. Eur. are used. In any case, lacking a finished product will not allow the manufacturer to submit an MAA nor is the product in question a pharmaceutical product. The primary mode of action of such a product remains with a medical device and thus, the medical device regulatory pathway needs to be followed including the quality aspects of the pharmaceutical constituent part according to the MPD 2001/83/EC.
Device Drug Combination Products
The other part of a combination product in our discussion, is the medicinal product. A medicinal product is regulated according to the Medicinal Product Directive MPD 2001/83/EC. To obtain a market license for a finished product, an active pharmaceutical ingredient (API) has to be formulated into a drug product and packaged accordingly to form the finished product. Whenever there is a finished product subject to market authorization, a market authorization application is to be submitted to the competent authorities must be done. Once reviewed and cleared, the competent authority grants the market authorization and the manufacturer becomes the market authorization holder (MAH). Only finished products can obtain a market authorization
A drug product constituent part is combined with a medical device to form a medical device combination product. Such products have a primary mode of action being physical and thus, are not a medicinal product. Therefore, these products undergo Notified Body review, based on the Technical Documentation, to obtain the EC Certificate to allow the manufacturer to CE mark the product. The Notified Body, not in charge to review the drug part of the drug product constituent part will ask the market applicant to liaise with a Competent Authority for the pharmaceutical review. The review of the conformity of a drug product constituent part in a Combination Product by a Competent Authority is called “Consultation Procedure”. Such a Consultation Procedure has to be submitted together with the Technical Documentation.

Device Drug Combination Products, per EU regulation, need to follow the Consultation Procedure.
How Avanti Europe can help
Avanti Europe’s Experts have a decade-long track record and expertise in consulting and hands-on working in process implementation in the Pharmaceutical, Cosmetical, and Medical Device industry. Our experts support your company with hands-on workforce and support in combination products’ risk-based process design, documentation, and training for the company staff. Visit our online shop for checklists and other services.